Ketamine as a Rapid Onset Antidepressant
The discovery of ketamine as a rapid-onset antidepressant and further exploration into the processes involved could significantly impact treatment for patients as well as public health worldwide.
Table of Contents
Major Depressive Disorder: A Global Concern
Major Depressive Disorder (MDD) impacts over 264 million people. This makes it the leading cause of disability globally. But, issues have been raised about how effective conventional treatments are. For instance, a large study found that less than a third of patients had depression ease after four months on standard antidepressants. These treatments also take a while to work, which can lead to higher rates of illness and even death during this period. This shows the need for alternatives, such as the fast-acting antidepressant ketamine.
Glutamate: The Powerful Neurotransmitter
Glutamate is a powerful neurotransmitter in our brain. It helps our neurons fire more easily. It activates various groups of receptors, including NMDA, AMPA, and kainate receptors. When activated, these channels allow ions to flow into cells, leading to internal signals being sent. AMPA receptors, in particular, manage most of the fast reactions at synapses and contribute to learning, memory, and brain protection processes.
The Role of Glutamate in MDD
Changes in the glutamate system have been noticed in people with MDD. Studies suggest that the glutamate system plays a key role in mood regulation. Moreover, post-mortem and genetic studies have shown abnormalities in the glutamate system in individuals with MDD, indicating the significant role it might play in the disorder. However, the exploration of glutamate in mood disorders has remained stagnant for some time.
Ketamine and Its Mechanism
How Ketamine Works
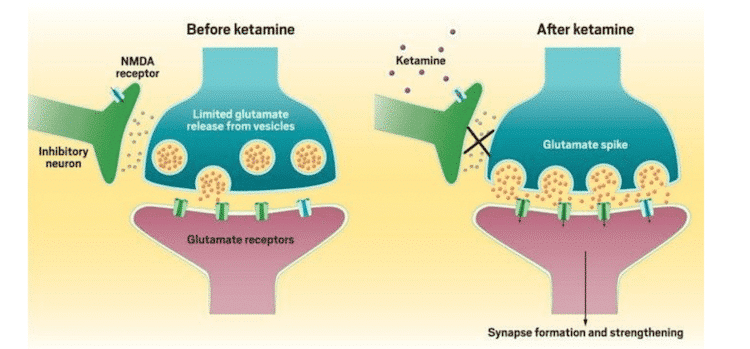
Ketamine is an NMDA antagonist. It works by blocking the NMDA receptor and triggering a significant glutamate release. This results in favoring AMPA receptors, which strengthen synapses. Interestingly, long-term use of standard antidepressants also increases AMPA receptor surface levels. These findings suggest that the strengthening of synapses by AMPA could contribute to the quick antidepressant effects seen with ketamine.
Ketamine as a Treatment
Ketamine (dl2-(o-chlorophenyl)-2-(methylamino) cyclohexanone hydrochloride) is an NMDA antagonist (Harrison & Simmonds, 1985) and a derivative of PCP. Its primary method of action is blocking the NMDA receptor at the PCP site within the ionotropic channel. Simultaneously, it induces a substantial presynaptic release of glutamate by increasing the firing rate of glutamatergic neurons. (Moghaddam, Adams, Verma, & Daly, 1997). This increase in glutamate release then favors AMPA receptors over NMDA receptors because the latter is blocked by ketamine, causing a greater throughput through the former and AMPA-mediated synapse strengthening.
Chronic treatment with standard antidepressants has also been shown to enhance AMPA receptor surface levels (Du et al., 2004; Du et al., 2007). Taken together, these findings suggest that the strengthening of synapses mediated by AMPA may be involved in the early antidepressant effects seen with ketamine, while intracellular signalling cascades that activate AMPA receptors from monoaminergic regulation modulate the long-term antidepressant effects of standard antidepressants.
In light of these discoveries, ketamine has been studied for MDD treatment. One study found that a single sub-anesthetic dose of ketamine had a quick and lasting antidepressant effect with no serious side effects. Over 70% of patients responded within 24 hours, and 35% still responded after a week. These response rates are similar to those achieved with standard antidepressants but after a longer period.
MDD, Alcoholism, and Ketamine
Recent studies have also been interested in the link between MDD, alcoholism, and the glutamate system. For instance, one study found that people with MDD and a family history of alcohol dependence responded better to ketamine treatment than those without such a history. The reasons for this are not entirely clear, but genetic alterations in NMDA receptor subunits might play a role.
Concerns with Ketamine Treatment
Side Effects and Risks
However, there are concerns about misuse and abuse of ketamine, as we’ve seen with other drugs like benzodiazepines and stimulants. A single case report suggested that repeated use of ketamine might lead to tolerance, meaning its antidepressant effects may decrease over time. There are also risks of psychosis, dissociative episodes, and severe emotional distress with repeated use. Moreover, ketamine’s side effects like sedation and psychotomimetic effects could limit its widespread use.
The Future of Antidepressant Treatment
Still, there is a significant gap in treatment as standard antidepressants take weeks to work fully, leaving patients vulnerable. The low rates of full recovery and frequent relapses point to the need for better antidepressants. Ketamine has shown a rapid and sustained antidepressant effect, suggesting the possibility of similar drugs in the future. Understanding how ketamine works could significantly improve treatment for patients and public health worldwide.
Alterations in the glutamatergic system have been observed in the central nervous system (CNS, CSF, and brain tissue) as well as the periphery in subjects with MDD (Sanacora et al., 2008). In fact, many studies support a critical role for the glutamatergic system in the pathophysiology of MDD, as it is believed to be a key target in mood regulation (Maeng & Zarate, 2007; Sanacora et al., 2008; Zarate, Quiroz, Payne, & Manji, 2002). Similarly, post-mortem and genetic studies support the role of glutamatergic system dysfunction in MDD, showing increased levels of glutamate and decreased levels of AMPA receptor subunits have been found in the prefrontal cortex of individuals with MDD (Beneyto & Meador-Woodruff, 2006; Hashimoto, Sawa, & Iyo, 2007; Scarr, Pavey, Sundram, MacKinnon, & Dean, 2003). Further, reduced NMDA receptor binding and subunit expression have also been found in the temporal and two frontal brain regions of subjects with MDD (Choudary et al., 2005; Nudmamud-Thanoi & Reynolds, 2004). Early reports also describe the action of antidepressants on glutamatergic receptors and the antidepressant-like effects of NMDA antagonists in animal models (Manji et al., 2003). However, for unclear reasons, exploration of glutamate in mood disorders remained stagnant until recently.
Yet, the fact remains that monoaminergic antidepressants take weeks to achieve their full effect, leaving patients receiving these medications particularly vulnerable. This, along with low rates of remission and frequent relapses are issues that need to be tackled with the next generation of antidepressants. Ketamine is a good proof of concept tool to develop biomarkers to further our understanding of the neuropsychological mechanisms of depression and what aspects are important in the development of future pharmacological treatments. It demonstrates a consistently reproducible antidepressant effect within a short period of time, showing that similar agents that can induce rapid and sustained effects after repeated doses is possible. The discovery of the rapid antidepressant effects of ketamine and further exploration into the processes involved could significantly impact treatment for patients as well as public health worldwide.
Related Articles
- Check out Frshminds’ Guide to Ketamine Clinics to learn about ketamine in order to select a clinic that meets your needs.
- 5 Things You Should Know Before Starting Ketamine Infusions for Bipolar Depression
- The Secret to Getting Health Insurance to Cover Ketamine Therapy
- Your First Ketamine Therapy Session: What to Expect
- Ketamine as a Rapid Onset Antidepressant
- A Ketamine Clinic Near Me: Roots Behavioral Health
- A Ketamine Clinic Near Me: The Infusion Clinic of Ocala
- A Ketamine Clinic Near Me: Dr Ken Starr in Arroyo Grande
- Ketamine Assisted Psychotherapy (KAP): Demographics, Data and Outcomes
- Meet ‘Sarah’, She Uses Ketamine Infusion Therapy for Depression
- Talking with a Ketamine Infusion Doctor: Dr. Franklin
References
Beneyto, M., & Meador-Woodruff, J. H. (2006). Lamina-specific abnormalities of AMPA receptor trafficking and signaling molecule transcripts in the prefrontal cortex in schizophrenia. Synapse, 60(8), 585-598. https://doi.org/10.1002/syn.20329
Boyce-Rustay, J. M., & Holmes, A. (2006). Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit.Psychopharmacology, 187(4), 455-466. https://doi.org/10.1007/s00213-006-0448-6
Britt, G. C., & McCance-Katz, E. F. (2005). A brief overview of the clinical pharmacology of “Club drugs”. Substance Use & Misuse, 40(9-10), 1189-1201. https://doi.org/10.1081/ja-200066730
Choudary, P. V., Molnar, M., Evans, S. J., Tomita, H., Li, J. Z., Vawter, M. P., Myers, R. M., Bunney, W. E., Akil, H., Watson, S. J., & Jones, E. G. (2005). Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proceedings of the National Academy of Sciences, 102(43), 15653-15658. https://doi.org/10.1073/pnas.0507901102
Dillon P, Copeland J, Jansen K. Patterns of use and harms associated with non-medical ketamine use. Drug Alcohol Depend 2003;69:23–28. [PubMed: 12536063]
Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST, et al. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci 2004;24(29):6578–6589. [PubMed: 15269270]
Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, et al. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology 2007;32(4):793–802. [PubMed: 16936714]
Entsuah, A. R., Huang, H., & Thase, M. E. (2001). Response and remission rates in different subpopulations with major depressive disorder administered Venlafaxine, selective serotonin reuptake inhibitors, or placebo. The Journal of Clinical Psychiatry, 62(11), 869-877. https://doi.org/10.4088/jcp.v62n1106
Green, S. M., Rothrock, S. G., Lynch, E. L., Ho, M., Harris, T., Hestdalen, R., Hopkins, G., Garrett, W., & Westcott, K. (1998). Intramuscular ketamine for pediatric sedation in the emergency department: Safety profile in 1,022 cases. Annals of Emergency Medicine, 31(6), 688-697. https://doi.org/10.1016/s0196-0644(98)70226-4
Harrison, N. L., & Simmonds, M. A. (1985). Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. British Journal of Pharmacology, 84(2), 381-391. https://doi.org/10.1111/j.1476-5381.1985.tb12922.x
Hashimoto, K., Sawa, A., & Iyo, M. (2007). Increased levels of glutamate in brains from patients with mood disorders. Biological Psychiatry, 62(11), 1310-1316. https://doi.org/10.1016/j.biopsych.2007.03.017
Ku, Y. H. (2020, January 15). Ketamine’s primary mechanism of action [Diagram]. Chemical and Engineering News. https://cen.acs.org/biological-chemistry/neuroscience/Ketamine-revolutionizing- antidepressant-research-still/98/i3
Liebrenz, M., Stohler, R., & Borgeat, A. (2009). Repeated intravenous ketamine therapy in a patient with treatment-resistant major depression. The World Journal of Biological Psychiatry, 10(4-2), 640-643. https://doi.org/10.1080/15622970701420481
Machado-Vieira, R., Salvadore, G., Luckenbaugh, D. A., Manji, H. K., & Zarate, C. A. (2008). Rapid onset of antidepressant action. The Journal of Clinical Psychiatry, 69(6), 946-958. https://doi.org/10.4088/jcp.v69n0610
Maeng, S., & Zarate, C. A. (2007). The role of glutamate in mood disorders: Results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Current Psychiatry Reports, 9(6), 467-474. https://doi.org/10.1007/s11920-007-0063-1
Malinow, R., & Malenka, R. C. (2002). AMPA receptor trafficking and synaptic plasticity. Annual Review of Neuroscience, 25(1), 103-126. https://doi.org/10.1146/annurev.neuro.25.112701.142758
Manji, H. K., Quiroz, J. A., Sporn, J., Payne, J. L., Denicoff, K., A. Gray, N., Zarate, C. A., & Charney, D. S. (2003). Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-Treat depression. Biological Psychiatry, 53(8), 707-742. https://doi.org/10.1016/s0006-3223(03)00117-3
Moghaddam, B., Adams, B., Verma, A., & Daly, D. (1997). Activation of Glutamatergic Neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to Dopaminergic and cognitive disruptions associated with the prefrontal cortex. The Journal of Neuroscience, 17(8), 2921-2927. https://doi.org/10.1523/jneurosci.17-08-02921.1997
Neuroscientifically Challenged. (2018, April 13). NMDA and AMPA receptors [Graphic]. Youtube. https://www.youtube.com/watch?v=29QfkTjIWHU&ab_channel=Neuroscientifi callyChallenged
Nudmamud-Thanoi, S., & Reynolds, G. P. (2004). The NR1 subunit of the glutamate/NMDA receptor in the superior temporal cortex in schizophrenia and affective disorders. Neuroscience Letters, 372(1-2), 173-177. https://doi.org/10.1016/j.neulet.2004.09.035
Phelps, L. E., Brutsche, N., Moral, J. R., Luckenbaugh, D. A., Manji, H. K., & Zarate, C. A. (2009). Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biological Psychiatry, 65(2), 181-184. https://doi.org/10.1016/j.biopsych.2008.09.029
Sanacora, G., Zarate, C. A., Krystal, J. H., & Manji, H. K. (2008). Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nature Reviews Drug Discovery, 7(5), 426-437. https://doi.org/10.1038/nrd2462
Scarr, E., Pavey, G., Sundram, S., MacKinnon, A., & Dean, B. (2003). Decreased hippocampal NMDA, but not kainate or AMPA receptors in bipolar disorder. Bipolar Disorders, 5(4), 257-264. https://doi.org/10.1034/j.1399-5618.2003.00024.x
Schumann, G., Johann, M., Frank, J., Preuss, U., Dahmen, N., Laucht, M., Rietschel, M., Rujescu, D., Lourdusamy, A., Clarke, T., Krause, K., Dyer, A., Depner, M., Wellek, S., Treutlein, J., Szegedi, A., Giegling, I., Cichon, S., Blomeyer, D., … Mann, K. (2008). Systematic analysis of Glutamatergic Neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Archives of General Psychiatry, 65(7), 826. https://doi.org/10.1001/archpsyc.65.7.826
Thase, M. E., Haight, B. R., Richard, N., Rockett, C. B., Mitton, M., Modell, J. G., VanMeter, S., Harriett, A. E., & Wang, Y. (2005). Remission rates following antidepressant therapy with Bupropion or selective serotonin reuptake inhibitors. The Journal of Clinical Psychiatry, 66(08), 974-981. https://doi.org/10.4088/jcp.v66n0803
Trivedi, M. H., Rush, A. J., Wisniewski, S. R., Nierenberg, A. A., Warden, D., Ritz, L., Norquist, G., Howland, R. H., Lebowitz, B., McGrath, P. J., Shores-Wilson, K., Biggs, M. M., Balasubramani, G. K., & Fava, M. (2006). Evaluation of outcomes with Citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. American Journal of Psychiatry, 163(1), 28-40. https://doi.org/10.1176/appi.ajp.163.1.28
Zarate CA, Quiroz J, Payne J, Manji HK. Modulators of the glutamatergic system: implications for the development of improved therapeutics in mood disorders. Psychopharmacol Bull. 2002 Autumn;36(4):35-83. PMID: 12858143
Zarate, C. A., Singh, J. B., Carlson, P. J., Brutsche, N. E., Ameli, R., Luckenbaugh, D.A., Charney, D. S., & Manji, H. K. (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry, 63(8), 856. https://doi.org/10.1001/archpsyc.63.8.856

Comments