
5 Things You Should Know Before Starting Ketamine Infusions for Bipolar Depression
If you are considering ketamine infusions for bipolar depression, you should know that according to a study published in the journal Expert Opinion on Drug Safety, ketamine is generally well tolerated and safe when administered mainly as an acute treatment (Rodrigues NB, McIntyre RS, Lipsitz O, et al. Safety and tolerability of IV ketamine in adults with major depressive or bipolar disorder: results from the Canadian rapid treatment center of excellence. Expert Opin Drug Saf. doi: 10.1080/14740338.2020.1776699), but before you talk to your doctor about it, here are 5 things you should know.
Table of Contents
Ketamine and Bipolar Depression
Writing in the September 2015 edition of The Lancet, Tony Kirby’s “Ketamine for Depression: the highs and lows” best articulates the pros and cons of off-label ketamine therapy to treat depression. After speaking with ketamine pioneers like Dr David Feifel, a psychiatrist at the University of California, San Diego and one of the first clinicians to use ketamine off-label as well as Dominic Sisti, an assistant professor in the Department of Medical Ethics and Health Policy at the Perelman School of Medicine, University of Pennsylvania, Kirby sums up the key ethical dilemma facing both patients and practitioners as “balancing between prescribing ketamine off-label to patients with depression against making all patients wait until ketamine or a derivative is licensed for depression”. If you have only recently started to consider ketamine as means to manage manic depression, it is important to understand and explore:
- The potential of treating bipolar depression disorders with ketamine type drugs;
- The history of ketamine;
- How ketamine works within the human nervous system; and,
- The role of glutamate and neuroplasticity.
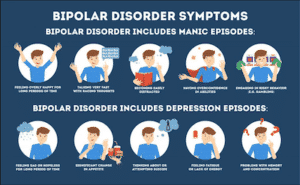
Bipolar disorder, formerly called manic depression, is a mental health condition that causes extreme mood swings that include emotional highs (mania or hypomania) and lows (depression). Symptoms can include an extremely elevated mood called mania as well as episodes of depression. People with bipolar disorder may have trouble managing everyday life tasks at school or work, or maintaining relationships. There’s no cure, but there are many treatment options, including using ketamine infusions to manage bipolar depression.
1. History of Ketamine for Depression
During the 1950’s and 60s, ketamine and a number of related drugs were created in the search for a drug with both anesthetic and analgesic effects. First administered to humans in 1964, patients described their ketamine experience as a “feeling of floating in outer space and having no feelings in the limbs” and based on this, ketamine became known as a ‘dissociative anaesthetic, characterized as the “electrophysiological and functional dissociation between thalamocortical and limbic systems. Currently used in clinical and emergency settings as an anesthetic during short procedures, ketamine is utilized in the treatment of numerous forms of chronic pain (postoperative, neuropathic and cancer). Additionally, there has been research into using ketamine in the management of bipolar depression, major depressive disorder (MDD) and treatment resistant depression.
The Effectiveness of Ketamine in Managing Depressive Disorders
Ketamine has been studied for major depressive and bipolar disorders. It has consistently shown better outcomes than placebos or pharmaceutical solutions. It has proven to reduce depressive symptoms in the short-term after administration, to lower the frequency and intensity of depressive or suicidal thoughts.
2. Ketamine Infusion for Bipolar Depression
The infusion of low doses of ketamine for bipolar depression has been studied extensively, and the findings are pretty consistent. Multiple studies have confirmed the effectiveness of a single infused dose of ketamine for bipolar depression that has been otherwise resistant to treatment. It also seemed to work well in concert with mood stabilizers, yielding a meaningful antidepressant effect.
3: Anti-suicidal Effect of Ketamine in Bipolar Depression
A single ketamine infusion has been proven to cause a significant reduction in suicidal thoughts for people dealing with treatment-resistant depression. The benefits tended to accrue within a day of receiving the dose. This is much faster than the action from any pharmaceutical alternative. Exactly why ketamine works so well is still not entirely understood.
4. Understanding the Nervous System
Before diving into how ketamine mediates depression, it is important to understand a little bit of the nervous system structure and how it functions. For our purposes, we are going to limit ourselves to some basic neurological/brain structures, functions and mechanisms so that we get a better understanding of how ketamine works in the human body. At a high level, the human nervous system contains two types of cells: neurons and glial cells. While important, right now all you need to know about glial cells is that they:
- Protect and hold neurons in place;
- Supply nutrients to neurons;
- Insulate neurons with myelin, a fatty substance wrapped around axons that increases the speed and efficiency at which action potentials propagate along the nerve; and
- Are resident immune cells within the central nervous system.
The Neuron
The fundamental building block of the human nervous system is a neuron, a specialized cell that transmits electrical nerve impulses in responses to neurotransmitters. Neurons have three basic components, the dendrite, the soma and the axons. With the naked eye, you see the nervous system as bundles of nerve fibers, the axon portions of neurons, that spread out from the brain/spinal cord and connect to every part of the body. Neurons transmit/propagate nerve impulses by changing their membrane potential, which is the difference in electric potential between the interior and the exterior of the cell.
The Synapse: Where All The Action Is
Neurons communicate with other cells via a synapse, a cellular membrane to cellular membrane junction that facilitates the transmission of electrical or chemical signals to another neuron or the target cell. At a synapse, the presynaptic neuron comes into close approximation with the postsynaptic cell. The postsynaptic cell membrane contains an extensive array of receptors, protein based cellular structures, that initiate the signaling process. Receptors attach to chemical messengers, like hormones and neurotransmitters, in order to either:
- Cause some form of cellular/tissue response/chemical pathway (eg. like a change in the polarity of the cell wall), or,
- Inhibit a cellular response (eg limiting production of a cellular metabolite).
Which change the electrical potential of a nerve cell for the purpose of coordinating a biological action. While different types of receptors are found in most cells, each receptor will only bind with specific chemicals, much like how a lock only works with specific keys.
5. Ketamine for Bipolar Depression: Understanding Glutamate
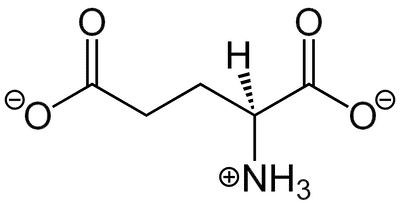
Glutamate, the most abundant excitatory neurotransmitter in the brain, is a powerful neurotransmitter responsible for sending signals between nerve cells, and at normal levels, plays an important role in learning and memory.
The Role of Glutamate in the Brain
Glutamate plays a large role in learning, and memory via a mechanism known as synaptic plasticity, a cellular process that results in lasting changes in the efficacy of neurotransmission. Neuroplasticity is a way for the brain to fine-tune itself for efficiency and happens continually throughout a person’s life as your brain develops with exposure to new data and experiences. It involves altering neural synapses and pathways, neurons, vascular cells, and glial cells. Another part of this process is synaptic pruning, which is the brain’s way of deleting the neural connections that are no longer necessary or useful and strengthening the necessary ones. The human brain decides which connections to prune based on each individuals’ life experiences and how recently connections have been used; neurons that grow weak from underuse die off through the process of apoptosis.
From a more clinical point of view, neuroplasticity, refers to variability in the strength of a signal transmitted through a synapse as a result of:
- Changes in intracellular signaling cascades and gene regulation,
- Modifications of synaptic number and strength,
- Variations in neurotransmitter release, and,
- Modeling of axonal and dendritic architecture, and, in some areas of the central nervous system, the generation of new neurons.
Neural plasticity, a fundamental mechanism of neuronal adaptation, is thought to be disrupted in those who suffer with depression. The changes in neural plasticity induced by stress and other negative stimuli play a significant role in the onset and development of depression. Antidepressant treatments have also been found to exert their antidepressant effects through regulatory effects on neural plasticity. However, while the detailed mechanisms of neuroplasticity in depression still remain unclear, there is general consensus in the role of glutamate in the process.
Glutamate is abundant in the nervous system and is the:
- Body’s most prominent neurotransmitter,
- Brain’s main excitatory neurotransmitter, and
- Precursor for GABA, the brain’s main inhibitory neurotransmitter.
First identified as a neurotransmitter in 1959, numerous studies demonstrated the role of the glutamatergic system in psychiatric disorders; Initial studies showed elevated glutamate levels in serum and cerebrospinal fluid from patients with mood disorders. The function/dysfunction of the glutamatergic system is hypothesized to play a role in mood disorders in two ways:
- Elevated glutamate: Abnormally high concentrations of glutamate may overexcite the receiving neuron.
- Postsynaptic glutamate sensitivity: Glutamate receptors on postsynaptic neurons can be oversensitive, such that a lower concentration threshold of glutamate molecules is needed to excite that cell.
As a result, glutamate activated cells become overactive, which leads to cell damage and/or death. While not simple to interpret, evidence from a range of studies demonstrate the role of the glutamatergic system in major depressive disorders, including bipolar depression and treatment resistant depression. Nuclear resonance studies in those with mood disorders demonstrate altered glutamate level in various regions of the brain; elevated in the occipital cortex and decreased in the anterior cingulate cortex appear to be the most consistent findings in nuclear magnetic resonance spectroscopy. Studies of postmortem tissue from brains of psychiatric patients noted that patients with depression had abnormally high expression levels of many genes that regulate the glutamate system. As research linked mood disorders with the impaired neuroplasticity in various regions of the central nervous system, medical professionals hypothesized that medications capable of increasing resilience to glutamate and modulating its role in neuroplasticity, are promising therapeutics in the treatment of mood disorders.
Is the Glutamate Receptor the Key To Managing Depression?
Ketamine is believed to exert its antidepressant properties through N-methyl-D-aspartate receptor (the NMDA receptor) antagonism. A receptor antagonist is a chemical that unlike an agonist, dampens a biological response by binding to a specific receptor.
The NMDA receptor is one of three types of glutamate receptors found on nerve cells and is activated by the binding action of glutamate. Receptor activation allows positively charged ions to flow through the nerve cell membrane. As previously discussed, research demonstrates that glutamate and the NMDA receptor is very important in neuroplasticity and memory.
Ketamine’s Mechanism of Action in Treating Depression
Ketamine’s inhibition of the N-methyl-D-aspartate glutaminergic receptors has been linked to analgesic, dissociative, and neuroprotective effects. Ketamine also interacts with opioid receptors at high plasma concentrations, causing additional analgesic effects. Ketamine is thought to exert its antidepressant effects through N-methyl-D-aspartate receptor antagonism and possible inhibitory effects on the norepinephrine and serotonin transporter function. Combining all these effects, ketamine administration causes anesthesia, analgesia, tachycardia, increased blood pressure, impaired memory and cognitive function, and visual changes in a dose-dependent manner.
Related Articles
- Check out Frshminds’ Guide to Ketamine Clinics to learn about ketamine in order to select a clinic that meets your needs.
- 5 Things You Should Know Before Starting Ketamine Infusions for Bipolar Depression
- The Secret to Getting Health Insurance to Cover Ketamine Therapy
- Your First Ketamine Therapy Session: What to Expect
- Ketamine as a Rapid Onset Antidepressant
- A Ketamine Clinic Near Me: Roots Behavioral Health
- A Ketamine Clinic Near Me: The Infusion Clinic of Ocala
- A Ketamine Clinic Near Me: Dr Ken Starr in Arroyo Grande
- Ketamine Assisted Psychotherapy (KAP): Demographics, Data and Outcomes
- Meet ‘Sarah’, She Uses Ketamine Infusion Therapy for Depression
- Talking with a Ketamine Infusion Doctor: Dr. Franklin

Comments