
Ketamine Assisted Psychotherapy (KAP): Demographics, Data and Outcomes
By now you’ve probably heard a bit about the potential benefits of ketamine assisted psychotherapy. Read on as Frshminds’ Emily Frewster takes you behind the curtain to explore a bit of the research into ketamine assisted psychotherapy.
Ketamine Assisted Psychotherapy (KAP): Patient Demographics, Clinical Data and Outcomes in Three Large Practices Administering Ketamine with Psychotherapy
The article below is part of Frshmind’s “Psychedelic Science Snapshot Series” where Frshminds reviews the latest in psychedelic research.
Original authors: Jennifer Dore, Brent Turnipseed, Shannon Dwyer, Andrea Turnipseed, Julane Andries, German Ascani, Celeste Monnette, Angela Huidekoper, Nicole Strauss & Phil Wolfson
Summarized by: Emily Fewster
Introduction
Recently, groundbreaking clinical research on the therapeutic use of psychedelic substances such as psilocybin, MDMA, and ketamine has been gaining interest. Originating as an anesthetic in the 1960s, early explorations of the drug identified a very specific side effect – patients would experience hallucinations, often so vivid that they were terrified. It wasn’t until the 1970’s when ketamine’s potential was extended from the physical to the psychological, with advancement in the research appearing in far flung locales from Argentina to Russia. After a lengthy hiatus in research, by the 1990s the National Institute of Mental Health rediscovered ketamine as an antidepressant while seeking alternatives to SSRIs / SNRIs.
Historical Psychedelic Research
Some of the earliest research into ketamine as an antidepressant sought to find a way to administer the drug so that its therapeutic qualities could be delivered without the accompanying hallucinations. While it did work to a certain degree, the benefits were not long-lasting. As we are discovering in respect of most psychedelic compounds, it is precisely the hallucinatory experiences which cause the most profound improvements. The antidepressant effects may be explained by ketamine’s primary interaction, disrupting the NMDA receptors in the glutamate system (which is the same way that synthetic opioids work on the body).
The Safety of Ketamine
With decades of emerging evidence being used in clinical settings, ketamine is now widely regarded as safe to use, even if self-administered at home (in a reduced dosage). There are some common, mild adverse effects including dizziness, drowsiness, elevated blood pressure, and not surprisingly, feeling disassociated from reality. These ‘adverse effects’ may be nothing more than the medicine doing its work. And there have been no cases of clearly negative physical or psychological effects (at least none that were persistent). And ketamine has not been associated with any material substance abuse issues. With all of this usage, even dosing regimens have become quite clear.
Establishing Ketamine Dosages
Over a course of Ketamine Assisted Psychotherapy (KAP) treatments, the therapist often chooses to escalate doses so that by the last sessions, the patient is receiving a full out-of-body experience. It is also clear that there are safe dosing strategies regardless of how ketamine is administered, whether nasally, under the tongue, or through an infusion.
The Effectiveness of Ketamine Assisted Psychotherapy
Whether a particular series of KAP sessions is effective depends on a variety of factors. The escalating dosage practice described above has been found through trial and error to produce the most significant and long lasting benefits, while also minimizing patient trepidation. This type of psychotherapy is intensive for both practitioners and their patients. Sessions often take 4 – 6 hours from start to finish. The empathetic bond between the patient and the therapist does have an impact on outcomes. Integration, the practice of drawing the insights gained from the KAP sessions into your conscious mind for regular recall and reinforcement, is also critical to the longevity of the benefits.
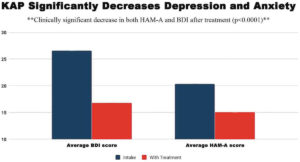
A recent study of the outcomes of KAP found statistically significant reductions in anxiety and depression. These effects were most acute when the patient has also had some type of developmental trauma. The number of KAP sessions and the duration of the entire course of treatment both appeared to play a direct role in the improvements noted. Those patients who had the most severe cases of these afflictions also reported the most considerable improvements.
It has become quite clear that the psychedelic/dissociative effects of the drug play a critical role in KAP’s positive effects on the patient. Competition from other current pharmacological treatments, like SSRI’s, have more significant side-effects and must be taken continuously or have their effects wear off.
KAP isn’t effective for everyone; there are cases of patients who appear to be immune to the effects. Patients with highly rigid personality structures, like OCD or a personality disorder, do not seem to garner the same long-lived benefits. But generally speaking, the results are very promising across the board.
Learn More About Ketamine
- Before Googling “Ketamine Treatment for Anxiety Near Me“…
- 5 Things You Should Know Before Starting Ketamine Infusions for Bipolar Depression
- The Secret to Getting Health Insurance to Cover Ketamine Therapy
- Your First Ketamine Therapy Session: What to Expect
- Ketamine as a Rapid Onset Antidepressant
- A Ketamine Clinic Near Me: Roots Behavioral Health
- A Ketamine Clinic Near Me: The Infusion Clinic of Ocala
- A Ketamine Clinic Near Me: Dr Ken Starr in Arroyo Grande
- Meet ‘Sarah’, She Uses Ketamine Infusion Therapy for Depression
- Talking with a Ketamine Infusion Doctor: Dr. Franklin
Intrigued by the potential of ketamine treatment? Uncover valuable insights by visiting Frshminds’ Guide to Ketamine Clinics, a comprehensive resource showcasing esteemed ketamine clinics in the USA plus a range of articles on all things related to ketamine treatment.
References
Chilukuri, H., R. Pothula, R. Pathapati, A. Manu, S. Jollu, and A. Shaik. 2014. Acute antidepressant effects of intramuscular versus intravenous ketamine. Indian Journal of Psychological Medicine (36/1):71–76. doi:10.4103/0253-7176.127258.
Collins, K., J. Murrough, A. Perez, D. Reich, D. Charney, and S. Mathew. 2010. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biological Psychiatry 67 (2):139–45. doi:10.1016/j.biopsych.2009.08.038.
Fontana, A. 1974. Terapia antidepresiva con ketamine. Acta psiquiatrica y psicologica deAmerica latina 20:32.
Griffiths, R., M. Johnson, W. Richards, B. Richards, U. McCann, and R. Jesse. 2011. Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology (Berl) 218 (4):649–65. doi:10.1007/s00213-011-2358-5.
Jaitly, V. 2013. Sublingual ketamine in chronic pain: Service evaluation by examining more than 200 patient years of data. Journal of Observational Pain Medicine 1/2 (2013).
Khorramzadeh, E., and A. Lofty. 1973. The use of ketamine in psychiatry. Psychosomatics 14:344–46. doi:10.1016/S0033-3182(73)71306-2.
Krupitsky, E., and A. Grinenko. 1997. Ketamine psychedelic therapy (KPT)—A review of the results of ten years of research. Journal of Psychoactive Drugs 29 (2):165–83.doi:10.1080/02791072.1997.10400185.
Krystal, J. H., L. P. Karper, J. P. Seibyl, G. K. Freeman, R. Delaney, J. D. Bremner, and D. S.Charney. 1994. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry 51 (3):199–214. doi:10.1001/archpsyc.1994.03950030035004.
Krystal, J. H. 2007. Ketamine and the potential role for rapid-acting antidepressant medications. Swiss Medical Weekly 137 (15–16):215–16.
Lara, D., L. Bisol, and L. Munari. 2013. Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. The International Journal of Neuropsychopharmacology 16 (9):2111–17. doi:10.1017/S1461145713000485.
Luckenbaugh, D. A., M. J. Niciu, D. F. Ionescu, N. M. Nolan, E. M. Richards, N. E. Brutsche, and C. Zarate. 2014. Do the dissociative side effects of ketamine mediate antidepressant effects. Journal of Affective Disorders 159:56–61. doi:10.1016/j.jad.2014.02.017.
Matthew, S., and C. Zarate, editors. 2016. Ketamine for Treatment Resistant Depression. Switzerl: Adis, Springer International.
McGirr, A., M. Berlim, D. Bond, M. Fleck, L. Yatham, and R. W. Lam. 2014. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychological Medicine. doi:10.1017/S0033291714001603.
Moghaddam, B., Adams, B., Verma, A., & Daly, D. (1997). Activation of Glutamatergic Neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to Dopaminergic and cognitive disruptions associated with the prefrontal cortex. The Journal of Neuroscience, 17(8), 2921-2927. https://doi.org/10.1523/jneurosci.17-08-02921.1997
Nour, M. M., L. Evans, D. Nutt, and R. L. Carhart-Harris. 2016, June 14. Ego-dissolution and psychedelics: Validation of the Ego-Dissolution Inventory (EDI). Frontiers in Human Neuroscience 10:269. doi: 10.3389/fnhum.2016.00269.eCollection2016.
Sanacora, G., Zarate, C. A., Krystal, J. H., & Manji, H. K. (2008). Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nature Reviews Drug Discovery, 7(5), 426-437. https://doi.org/10.1038/nrd2462
Sullivan, P. 2018. Using ketamine to treat addictions. Paper presented at the Kriya Conference, The Nueva School. Hillsborough, CA, November 4.
Van Der Kolk, B. 2014. The body keeps the score. London, UK: The Penguin Group. Wallach, J. 2018. Pharmacokinetics and pharmacodynamics of ketamine (and Related Compounds). Paper Presented at the Kriya Conference, The Nueva School. Hillsborough, CA, November 2.
Weber, F., H. Wulf, M. Gruber, and R. Biallas. 2004. S-ketamine and s-norketamine plasmaconcentrations after nasal and iv administration in anesthetized children. Paediatr†anaesth 14 (12):983–988.
Wieber, J., R. Gugle, J. Hengstmann, and H. Dengler. 1975. Pharmacokinetics of ketamine in man. Anaesthesist 24 (6):260–63.

Comments