
MDMA Assisted Psychotherapy for PTSD
MDMA Assisted Psychotherapy for PTSD is a relatively recent area of study. While reports in the 1970’s described the potential for MDMA to facilitate communication and connection between therapists and patients, the 80’s saw the recreational use of MDMA became popular, leading to its classification as a Schedule 1 controlled substance in 1985, and thus created barriers for research and therapy. Recently, there has been a groundswell of attitude change towards the therapeutic potential of MDMA.
Table of Contents
MDMA Assisted Psychotherapy for PTSD Treatment
The article below is part of Frshminds’ “Psychedelic Science Snapshot Series” where Frshminds reviews the latest in psychedelic research.
Original authors: Michael C. Mithoefer, Allison A. Feduccia, Lisa Jerome, Anne Mithoefer, Mark Wagner, Zach Walsh, Scott Hamilton, Berra Yazar-Klosinski, Amy Emerson, & Rick Doblin
Summarized by: Emily Fewster
Introduction: MDMA, Psychotherapy and PTSD
Post-traumatic stress disorder (PTSD) is often the result of a person’s experiencing a traumatic event. PTSD is characterized by symptoms such as negativity, detachment, hyper-reactivity, and having memories that are unwanted and hard to ignore (Koenen et al. 2017). PTSD is surprisingly common, with estimates of the number of sufferers ranging up to 5% globally (Kilpatrick et al. 2013; Koenen et al. 2017).
Psychotherapy is typically the first line of treatment for PTSD. But this is by no means a guaranteed remedy, and may or may not result in significant out-of-pocket expense. There are a few approved pharmaceutical remedies, but they are also imperfect, with perhaps half of all patients not responding to them, not to mention the potential side effects and the fact that they must be used indefinitely to bring lasting change.
History of MDMA and Psychotherapy
MDMA’s reputation as a party drug in the 1980’s meant that it was quite difficult to conduct clinical research into it’s efficacy. MAPS, the Multidisciplinary Association for Psychedelic Studies, a not-for-profit advocating for the normalization of psychedelics, conducted a study (of 6 other studies, drawn from Phase 2 trials) that sought to understand whether MDMA could be used in conjunction with psychotherapy to help alleviate PTSD. If the results were promising, MAPS’ advocacy would turn to the acceleration of the third phase of research in the studies. After demonstrating that MDMA was not intrinsically harmful or toxic to the patient, the studies turned their attention to the therapeutic benefits.
MDMA Assisted Psychotherapy – Groundbreaking Clinical Work
In the studies, doses of MDMA were given to the PTSD sufferers. Their therapy team would communicate with the patients, steer them through periods of introspective thinking, and encourage them to wear blindfolds and headphones to drive their sensory experience through music and visuals. This approach was thought to allow patients to face their trauma without becoming completely overwhelmed by the pain or anxiety such confrontation would cause. A steady series of integration sessions followed, with the same therapy team working with the patients to incorporate their experience into their lives.
Patient Assessments
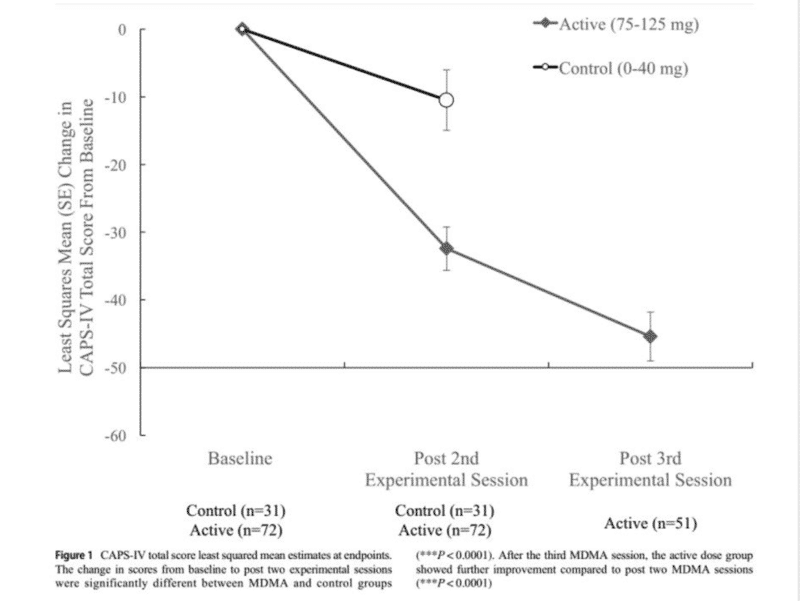
The improvements in the “Active Group” (patients who had received actual MDMA) and “Control” (who received placebos) were statistically significant and considerable. The active group collectively reported substantial decreases in “CAPS-IV” scores (essentially, the degree to which they experience PTSD symptoms. More than 50% of them no longer even met the definition of afflicted with PTSD. Truly, the impact of MDMA was real, and it was spectacular.
MDMA Assisted Psychotherapy Safety Outcomes
Safety was measured across a number of metrics – any reporting of side effects or unwanted outcomes, the potential for abuse of the drug after the trials were over, etc. Essentially the results showed that some mild or moderate side effects were often present, but they tended to dissipate within a week or less; these may include panic attacks and irritability. Importantly, while suicidal thoughts were reported by one member of the group prior to treatment, no reports emerged after the MDMA was administered. And critically, there were no instances of behavior that would indicate that the patients would seek to abuse MDMA now that the trial was over.
Advancing to Phase 3 Trials for MDMA-Assisted Psychotherapy
The results of the phase 2 trials were pretty definitive. Patients who had not responded to pharmaceutical treatments did respond to the MDMA, and were positively impacted. The successful results have, in fact, brought phase 3 trials to the fore. In this round of study, patients would receive additional doses of MDMA as much evidence is emerging that shows that additional doses drove even greater responses, and for longer durations. If successful once more, by 2022 the FDA may in fact recognize MDMA as a successful and legal treatment for PTSD, a long-awaited breakthrough for a substance that can help millions of people.
References
Beck, A. T., Steer, R. A., Ball, R., & Ranieri, W. F. (1996). Comparison of Beck depression Inventories-IA and-II in psychiatric outpatients. Journal of Personality Assessment, 67(3), 588-597. https://doi.org/10.1207/s15327752jpa6703_13
Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM (1995) The development of a clinician-administered PTSD scale. J Trauma Stress 8:75–90 Bradley, R., Greene, J., Russ, E., Dutra, L., & Westen, D. (2005). A multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry, 162(2), 214-227. https://doi.org/10.1176/appi.ajp.162.2.214
Brady, K., Pearlstein, T., Asnis, G. M., Baker, D., Rothbaum, B., Sikes, C. R., & Farfel, G. M. (2000). Efficacy and safety of Sertraline treatment of posttraumatic stress disorder. JAMA, 283(14), 1837. https://doi.org/10.1001/jama.283.14.1837
Camí, J., Farré, M., Mas, M., Roset, P. N., Poudevida, S., Mas, A., San, L., & De la Torre, R. (2000). Human pharmacology of 3,4-Methylenedioxymeth-amphetamine (“Ecstasy”): Psychomotor performance and subjective effects. Journal of Clinical Psychopharmacology, 20(4), 455-466.https://doi.org/10.1097/00004714-200008000-00010
de la Torre R, Farre M, Roset PN, Lopez CH, Mas M, Ortuno J, Menoyo E, Pizarro N, Segura J, Cami J (2000) Pharmacology of MDMA in humans. Ann N Y Acad Sci 914:225–237
Dorrington, S., Zavos, H., Ball, H., McGuffin, P., Rijsdijk, F., Siribaddana, S., Sumathipala, A., & Hotopf, M. (2014). Trauma, post-traumatic stress disorder and psychiatric disorders in a middle-income setting: Prevalence and comorbidity. British Journal of Psychiatry, 205(5), 383-389. https://doi.org/10.1192/bjp.bp.113.141796
Frith, C. H., Chang, L. W., Lattin, D. L., Walls, R. C., Hamm, J., & Doblin, R. (1987). Toxicity of Methylenedioxymethamphetamine (MDMA) in the dog and the rat. Toxicological Sciences, 9(1), 110-119. https://doi.org/10.1093/toxsci/9.1.110
Greer, G., & Tolbert, R. (1986). Subjective reports of the effects of MDMA in a clinical setting. Journal of Psychoactive Drugs, 18(4), 319-327. https://doi.org/10.1080/02791072.1986.10472364
Grob, C. S., Greer, G. R., & Mangini, M. (1998). Editors’ introduction: Hallucinogens at the turn of the century. Journal of Psychoactive Drugs, 30(4), 315-319. https://doi.org/10.1080/02791072.1998.10399707
Grob, C. S., Poland, R. E., Chang, L., & Ernst, T. (1996). Psychobiologic effects of 3,4-methylenedioxymethamphetamine in humans: Methodological considerations and preliminary observations. Behavioural Brain Research, 73(1-2), 103-107.https://doi.org/10.1016/0166-4328(96)00078-2
Grof S (2001) LSD psychotherapy, 4th edn. Multidisciplinary Association for Psychedelic Studies, Ben Lomond, CA
Gronwall, D. M. (1977). Paced auditory serial-addition task: A measure of recovery from concussion. Perceptual and Motor Skills, 44(2), 367-373. https://doi.org/10.2466/pms.1977.44.2.367
Harris, D. S., Baggott, M., Mendelson, J. H., Mendelson, J. E., & Jones, R. T. (2002). Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology, 162(4), 396-405. https://doi.org/10.1007/s00213-002-1131-1
Kibler, J. L., Joshi, K., & Ma, M. (2009). Hypertension in relation to posttraumatic stress disorder and depression in the US national comorbidity survey. Behavioral Medicine, 34(4), 125-132. https://doi.org/10.3200/bmed.34.4.125-132
Kilpatrick, D. G., Resnick, H. S., Milanak, M. E., Miller, M. W., Keyes, K. M., & Friedman, M. J. (2013). National estimates of exposure to traumatic events and PTSD prevalence usingdsm-ivanddsm-5Criteria. Journal of Traumatic Stress, 26(5), 537-547. https://doi.org/10.1002/jts.21848
Koenen KC, Ratanatharathorn A, Ng L, McLaughlin KA, Bromet EJ, Stein DJ, Karam EG, Meron Ruscio A, Benjet C, Scott K, Atwoli L, Petukhova M, Lim CCW, Aguilar-Gaxiola S, Al Hamzawi A, Alonso J, Bunting B, Ciutan M, de Girolamo G, Degenhardt L, Gureje O, Haro JM, Huang Y, Kawakami N, Lee S, Navarro-Mateu F, Pennell BE, Piazza M, Sampson N, Ten Have M, Torres Y, Viana MC, Williams D, Xavier M, Kessler RC (2017) Posttraumatic stress disorder in the world mental health surveys. Psychol Med 47:2260–2274
Lee, D. J., Schnitzlein, C. W., Wolf, J. P., Vythilingam, M., Rasmusson, A. M., & Hoge, C. W. (2016). Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: Systemic review and meta-analyses to determine first-line treatments. Depression and Anxiety, 33(9), 792-806. https://doi.org/10.1002/da.22511
Liechti, M. E., Gamma, A., & Vollenweider, F. X. (2001). Gender differences in the subjective effects of MDMA. Psychopharmacology, 154(2), 161-168. https://doi.org/10.1007/s002130000648
Marshall, R. P., Jorm, A. F., Grayson, D. A., & O’Toole, B. I. (2000). Medical-care costs associated with posttraumatic stress disorder in Vietnam veterans. Australian & New Zealand Journal of Psychiatry, 34(6), 954-962. https://doi.org/10.1080/000486700269
Mithoefer M (2017). A manual for MDMA-assisted psychotherapy in the treatment of posttraumatic stress disorder; Version 8. http://www.maps.org/research/mdma/mdma-research-timeline/4887-a-manual-for-mdma- assisted-psychotherapy-in-the-treatment-of-ptsd.
Mithoefer, M. C., Mithoefer, A. T., Feduccia, A. A., Jerome, L., Wagner, M., Wymer, J., Holland, J., Hamilton, S., Yazar-Klosinski, B., Emerson, A., & Doblin, R. (2018). 3,4-methylenedioxymethamphetamine (mdma)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. The Lancet Psychiatry, 5(6), 486-497. https://doi.org/10.1016/s2215-0366(18)30135-4
Mithoefer, M. C., Wagner, M. T., Mithoefer, A. T., Jerome, L., & Doblin, R. (2011). The safety and efficacy of ±3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. Journal of Psychopharmacology, 25(4), 439-452. https://doi.org/10.1177/0269881110378371
Nagy, L. M., Morgan, C. A., Southwick, S. M., & Charney, D. S. (1993). Open prospective trial of fluoxetine for posttraumatic stress disorder. Journal of Clinical Psychopharmacology, 13(2), 107???113. https://doi.org/10.1097/00004714-199304000-00004
Nichols, D. E. (1986). Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: Entactogens. Journal of Psychoactive Drugs, 18(4), 305-313. https://doi.org/10.1080/02791072.1986.10472362
Posner, K., Brown, G. K., Stanley, B., Brent, D. A., Yershova, K. V., Oquendo, M. A., Currier, G. W., Melvin, G. A., Greenhill, L., Shen, S., & Mann, J. J. (2011). The Columbia–suicide severity rating scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. American Journal of Psychiatry, 168(12), 1266-1277. https://doi.org/10.1176/appi.ajp.2011.10111704
Posner, K., Oquendo, M. A., Gould, M., Stanley, B., & Davies, M. (2007). Columbia classification algorithm of suicide assessment (C-CASA): Classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. American Journal of Psychiatry, 164(7), 1035-1043. https://doi.org/10.1176/ajp.2007.164.7.1035
Randolph, C. (1998). Repeatable battery for the assessment of Neuropsychological status. PsycTESTS Dataset. https://doi.org/10.1037/t15149-000
Scott, D. J., Stohler, C. S., Egnatuk, C. M., Wang, H., Koeppe, R. A., & Zubieta, J. (2008). Placebo and nocebo effects are defined by opposite opioid and Dopaminergic responses. Archives of General Psychiatry, 65(2), 220. https://doi.org/10.1001/archgenpsychiatry.2007.34
Steenkamp, M. M., Litz, B. T., Hoge, C. W., & Marmar, C. R. (2015). Psychotherapy for military-related PTSD. JAMA, 314(5), 489. https://doi.org/10.1001/jama.2015.8370
Tarrier, N., & Gregg, L. (2004). Suicide risk in civilian PTSD patients. Social Psychiatry and Psychiatric Epidemiology, 39(8). https://doi.org/10.1007/s00127-004-0799-4
Weathers, F. W., Keane, T. M., & Davidson, J. R. (2001). Clinician-administered PTSD scale: A review of the first ten years of research. Depression and Anxiety, 13(3), 132-156. https://doi.org/10.1002/da.1029

Comments